Yves here. Even though many readers will have seen the key details about the leaders in the vaccine horserace (and it is deeply distressing that not just the press but also policy-makers are fixated on speed), this article presents a good recap of the Oxford-AstraZeneca candidate. It also gives a good overview that friends and family members might find helpful.
I must note it fails to mention another downside of the messenger-RNA vaccines compared to the Oxford-AstraZeneca offering: distressing side effects. From CNBC yesterday:
Public health officials and drugmakers need to warn people that coronavirus vaccine shots may have some rough side effects so they know what to expect and aren’t scared away from getting the second dose, doctors urged during a meeting Monday with CDC advisors….
Dr. Sandra Fryhofer of the American Medical Association said both Pfizer’s and Moderna’s Covid-19 vaccines require two doses at varying intervals. As a practicing physician, she said she worries whether her patients will come back for a second dose because of the potentially unpleasant side effects they may experience after the first shot….
Participants in Moderna and Pfizer’s coronavirus vaccine trials told CNBCin September that they were experiencing high fever, body aches, bad headaches, daylong exhaustion and other symptoms after receiving the shots. While the symptoms were uncomfortable, and at times intense, the participants said they often went away after a day, sometimes sooner, and that it was better than getting Covid-19.
Both companies acknowledged that their vaccines could induce side effects that are similar to symptoms associated with mild Covid-19, such as muscle pain, chills and headache.
By Sanjay Mishra, Project Coordinator & Staff Scientist, Vanderbilt University Medical Center, Vanderbilt University. Originally published at The Conversation
The biopharmaceutical company AstraZeneca has released data on what is now the third promising vaccine candidate against COVID-19 – and it has several advantages over those of its competitors, Pfizer and Moderna.
On Monday, AstraZeneca released interim analysis of its phase 3 trial data of 23,000 volunteers from the U.K. and Brazil. These results show that the test vaccine is between 70% and 90% effective in stopping COVID-19, depending on the vaccine doses administered. Although less effective than the reported results from the Pfizer or Moderna COVID-19 vaccine candidates, this vaccine is still more effective than annual influenza vaccines that reduce the risk of flu by between 40% and 60%. Notably none of the vaccinated participants needed hospitalizations or reported severe disease.
Like most vaccine experts, I am intrigued by large differences in effectiveness between two tested dosages of AstraZeneca’s vaccine. Until March, I was developing vaccine candidates against Zika and dengue. Now I am coordinating a large crowd-sourced international effort to better understand the scope and severity of COVID-19 in cancer patients. The COVID-19 vaccine trials generally exclude most people with a history of cancer, so I am eagerly awaiting vaccine efficacy data for this risk group when these vaccines become widely available.
Intriguing Dose Response
AstraZeneca’s vaccine was originally planned to be given in two full doses, four weeks apart, as injections in the upper arm. A third of the volunteers were injected with a dummy saline placebo.
One of the few details that AstraZeneca released is that of 131 cases of COVID-19, only 30 cases were detected among 11,636 who were given the vaccine; 101 cases occurred among the volunteers who got the placebo. That suggests that the vaccine is 70% effective overall.
However, an error in the early stages of the trial meant that some participants received only a half-dose in the first round. In the group of 2,741 volunteers who received a lower dose of the vaccine candidate followed a month later by a full booster dose, the efficacy was 90%, according to AstraZeneca. The efficacy was only 62% among the 8,895 volunteers who received both full doses.
It is not clear why the half-dose plus the full dose sequence of the vaccine performs better than two full doses. One explanation could be that since the vaccine is based on a common, although nonhuman, cold virus, the immune system probably attacks and destroys it when the first dose is too large.
It is also possible that progressively increasing the dose more closely mimics a natural coronavirus infection. Beginning with a lower first dose might be a better way of kicking the immune system into action; then a stronger, more effective immune response occurs after the second full booster dose. Despite enormous progress in human immunology, scientists still don’t understand the best strategies for inducing protective immunity.
These results are based on the evaluation of about one-third of volunteers who are expected to participate in this trial, which is ongoing in other parts of the world and will enroll up to 60,000 people.
AstraZeneca will now seek approval from the FDA to also evaluate the half-dose protocol in the ongoing U.S. trial. The current trial involves 30,000 participants and is evaluating only the two full-dose regimen. AstraZeneca’s trials in the U.S. were halted temporarily in early September after a study participant in the U.K. fell ill, but resumed in the U.K., Brazil, South Africa and Japan.
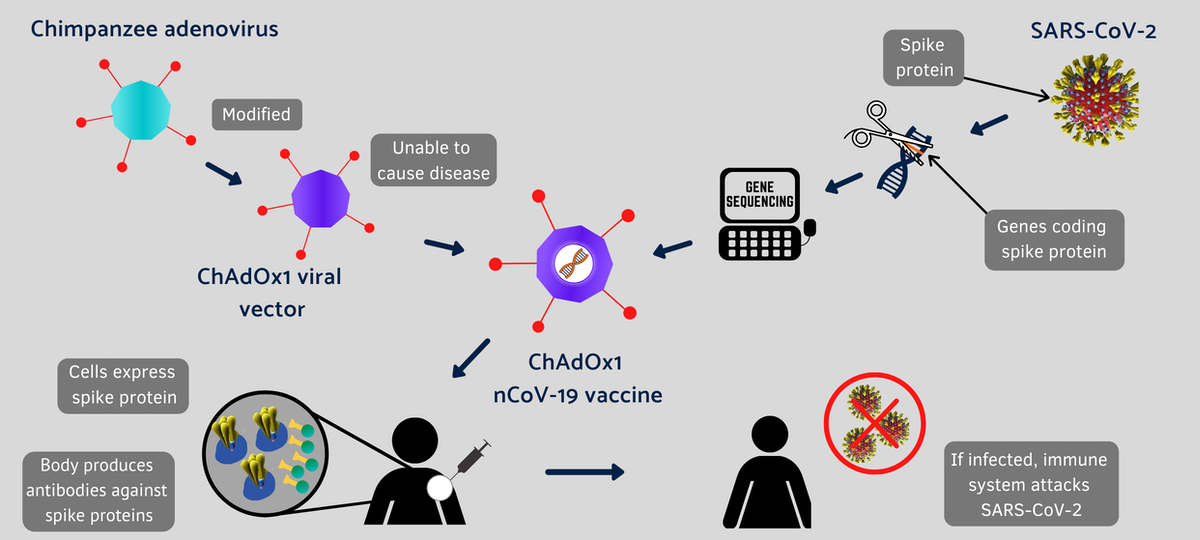
A Modified Chimpanzee Cold Virus
The Oxford-AstraZeneca vaccine is another example of a new strategy being used to rapidly develop vaccines against the coronavirus that has already infected over 58 million people worldwide.
A vaccine works as a primer to train the immune system against a pathogen.
Conventional vaccines are made by weakened viruses or by purifying their disease-causing protein, such as the spike protein, which decorates the surface of a coronavirus. But these methods can take decades to develop new vaccines. Coinvented by the University of Oxford and its spinout company, Vaccitech, this vaccine uses different molecular tools to provide a preview of the SARS-CoV-2 virus to the human body.
Instead of making weaker viruses, or delivering mRNA that encodes the spike protein, as Moderna and Pfizer did, the Oxford vaccine packs the DNA that codes for the spike protein in the shell of a genetically altered chimpanzee virus.
The original adenovirus causes common cold in chimpanzees and it rarely, if ever, infects humans. The virus is further modified to ensure that this chimp virus cannot grow in people. The AstraZeneca vaccine uses the modified virus as a vehicle to deliver the COVID-19-causing spike or S-protein of the SARS-CoV-2 virus.
Under the agreement with the University of Oxford, AstraZeneca is responsible for development, worldwide manufacturing and distribution of the vaccine.
This isn’t the first time that University of Oxford scientists have tried a vaccine using this harmless virus. Previously, it tested the concept against a closely related coronavirus that causes Middle East respiratory syndrome (MERS) in animal studies. So this time, soon after the sequence of the novel SARS-CoV-2 became available, the Oxford scientists retooled the chimp virus for a vaccine that induced robust immune response against SARS-CoV-2 in mice and rhesus macaques.
Not-So-Frigid Storage Requirement
Despite a somewhat later arrival, with less than the effectiveness claimed by its competitors, AstraZeneca’s vaccine might be favored because it can be stored, transported and handled at standard refrigerated conditions of between 36 and 46 degrees Fahrenheit for at least six months.
The competing mRNA vaccines by Moderna and Pfizer/BioNTech require ultracold temperatures for stability. So the AstraZeneca vaccine will be easier to use in normal clinics, especially in rural America and the developing world.
Another important advantage of the AstraZeneca vaccine, which is being tested in collaboration with a larger number of global sites, is that it should cost less because of AstraZeneca’s commitment to COVAX, a global initiative that aims to distribute low-cost vaccines to low- and middle-income countries. Pfizer and Moderna have not joined the COVAX initiative, but AstraZeneca has agreed to make the vaccine on a not-for-profit basis for the duration of the pandemic.
Wait and Watch
However, like all other candidate vaccines for COVID-19, AstraZeneca’s vaccine is also lacking in key details such as the breakdown in infections, the durability, or the efficacy in the different age groups of trial participants.
[Deep knowledge, daily. Sign up for The Conversation’s newsletter.]
For all the vaccine candidates, we have only preliminary data from a small number of infections, and none of the groups developing the COVID-19 vaccine candidates has so far published complete data. So it is difficult to fully assess the differences between them.
We will have to wait for more follow-up and longer-term data to evaluate the effectiveness of all the COVID-19 vaccines in finally getting the COVID-19 pandemic under control.


This whole vaccine protocol of taking two shots makes me wonder if scientists haven’t known for some time that two shots is the way to go for many diseases, but due to cost or the ability to get people to take even one shot, we end up with certain types of vaccines being far less effective. The most recent shingles vaccine, which is supposed to be incredibly effective, as well as very long lasting, regardless of age, is a two-part procedure–each shot is spaced 6 months apart, and the side effects of feeling miserable for a few days is not hidden from the patient.
Interesting question. Shingles 2-dose vaccine caused the worst reaction by far of any I’ve received. But worth it, from experiences of friends and family with bouts of shingles.
My understanding is that the two pneumonia shots each target a different set of strains of the disease.
I was told that the difference between the current flu shot designated for those over sixty-five and the shot designated for those under sixty-five is only that the former is a double dose of the same vaccine.
That seems a bit arbitrary, but those administering the shots at drug and grocery store pharmacies look closely at the birth date and won’t bend a day.
In the purely anecdotal department I couldn’t ditch a long-lingering cough, I think the residual of a Covid exposure abroad over a year ago. On a hunch I had the second pneumonia and second shingles shot at the same time, and the cough was gone the day after. Maybe the combo gunned my immune system, who knows? Anyway, that was my intent.
But regarding the Oxford-AstraZeneca shot, I’ll take three.
Yves Smith: Thanks for this assessment of the state of things. Here is an interview that I read yesterday that impressed me. It is in Italian, which means it gives another view of the same situation:
https://www.ilfattoquotidiano.it/2020/11/23/covid-ippolito-spallanzani-ecco-come-funzionera-il-vaccino-italiano-se-quando-tutto-sara-finito-avremo-una-sanita-migliore-i-45mila-morti-saranno-stati-onorati/6007913/
Giuseppe Ippolito is head of the Spallanzani infectious disease / virology center. They have the distinction of treating the first two cases in Europe–a Chinese couple from Wuhan who fell ill aboard a ship off the coast of Italy. They were in Spallanzani hospital for weeks but are now back in Wuhan, seemingly cured.
He also distinguishes between the Pfizer and Moderna tactic–mRNA–and the oldfashioned use of a repurposed virus, as in AstraZeneca. He notes that Johnson& Johnson and an Italian group called Reithera are following this second path. He says that Reithera has had some promising initial data, too, using this revised-virus approach.
There is a long paragraph in the middle in which he gives the state of play of drugs. It is interesting how he dismisses the antibody plasma infusion as simply too expensive. He notes that cortisone seems to have a good effect on outcomes.
So as in all scientific endeavors, there is good news, bad news, new data, and data that are swiftly becoming obsolete.
I would probably line up for this A-Z vaccine. It sounds like the technology is established using an adenovirus modified with the Cov-2 spike protein – and it does not use mRNA which is the big random variable imo. Even if this AZ jab needed to be kept frozen it would still be a safer bet simply because mRNA technology, regardless of how creative it is, is just too new to use to innoculate billions of people and if something goes wrong it will be the younger generations that will suffer the worst side-effects. Maybe they should reserve the AZ for the younger generations and offer the mRNA at-your-own-risk to the rest of us.
The final sentence of your comment is the “scientific process” in action.
It takes time and effort to understand cause and effect by trial (and sometimes error).
I’m kind of a dummy when it comes to this, but in these vaccine studies, after the volunteers get their shots (real vaccine or saline) do they just then send everyone out into the world to live their lives and then report back in a month or something like that? A week? More monitoring than that?
And I suppose that means that they would be sending them back into a society that now has mask mandates, and some form of “lockdown light” and social distancing, etc. Wouldn’t that have an effect on efficacy and the study results? That is, you aren’t sending people back into a ‘normal’ wide open society. So in a sense, the vaccine has a little bit of ‘help’ right now. Does this make any sense or am I way off?
I can address the US Pfizer phase 3 trial. We got two shots, one month apart, with a blood draw before the first. A month after the second shot, had another blood draw. There are blood draws scheduled in 6, 12, and 18 months. Once a week, we have to check in at a web site or via phone app and report any symptoms.
Other than that, yes, we are sent out into the world hopefully (in Pfizer’s view) to get infected (saline group) or not (vaccine group).
Thanks. Did you all wear masks etc? And are you in a town or city with mask and other mandates? I guess what I was also trying to get at above, is there a mitigation factor going on now in regards to being exposed, higher dose exposure than there otherwise might be because masks, social distancing, etc? And would this make a difference in the outcome of the effectiveness of a vaccine in these studies?
it would not make a difference only make it harder to see a difference if cases did not mount
The purpose of having a control group is to isolate any difference made by the vaccine. Both groups notionally have the same risk of being exposed to the virus whatever other mitigations are in place. The larger the test groups are the closer this comes to being true. So if the vaccinated group catch it less than the controls you can be confident the vaccine works.
Very good thanks!
My question is: how can this be a truly participant-blind study if the vaccine has side effects? If you get weird side effects, won’t you know you got the real vaccine?
Two cautionary points. The trial originally did not intend to use half-dose. The got the dosage wrong and only noticed after a bunch of people were given half-dose instead of full. They kept it in the trial because they didn’t want to waste already inoculated volunteers. So the statistics for the half-dose group is much worse than for the full dose group.
Also, the infection rate for the control group at this point is fairly low, a few percent I believe. This means that 80% efficiency and 90% efficiency are separated only by only a few extra infections. Standard deviation on these efficiencies are very high, ~20%. So the efficiency of the full dose vaccine has the range from 44% to 85%, while half-dose efficiency floor is ~65%.
These are all preliminary numbers as the trial is ongoing and as more subjects get infected the statistics will firm up. But this is important to stress are the efficiency numbers are very likely to be revised at some point in view of new data.
Even if they turn out to be the same once the statistical noise is ironed out, it’s still a win if it holds up because it means the vaccine would go further.
Not complaining here but thought you’d want to know: You’re getting a lot of timeouts from Cloudflare when I try to load your pages. Bizarrely the timeouts happen quickly and usually reloading does the trick.
I have experienced the same in recent days.
I had the same experience yesterday but today pages seem to load OK.
I have seen it elsewhere, too, like Fedsmith.com, so it may be a wider internet issue. It has been ongoing for a couple of weeks, for me, at least.
Just pulled up Wapo website. It had an editor’s note at the top saying widespread tech problems on the east coast may affect your access.
(Not sure what happened, my comment disappeared. Hope it doesn’t post twice now.)
Is the Covid pandemic being used as part of the “great reset”?
https://wrenchinthegears.com/
Alison McDowel nails the great reset. Under reported in the alt media, she has a clear idea where this going.
In her video, “The Fourth American Industrial Revolution Revealed” she discusses how we will become (are becoming) prisoners of technology.
https://youtu.be/ie33AbqWkq8
The Great Reset. Artificial intelligence, blockchain IDs, the internet of things, digital health passports (coming,) social credit scores (already here,) genetic profiling, neural programming, 5G, self driving cars, wearable/implantable technology . . ..
Virtual consumption.They’re turning human beings into a commodity.
Our rights will be controlled and individualized by a bio-security state and run on block chain. It will start by our government’s forcing us to have a blockchain security ID for health (pandemic) purposes and they will layer in education, housing and all government services.
Australia, working with Singapore is one of about 6 countries on the leading edge of the block chain bio security state. Their disability payment system is already in blockchain.
It is frightening, but not hopeless. As she said in the video above, “they wouldn’t want to control us if we weren’t powerful.”
RESIST!
Yesterday NPR interviewed Dr. Hotez co-director of vaccine development in Texas Children’s’ hospital. He went on at length about other Corona Virus pandemics/epidemics on the horizon indicating continual vaccinations with boosters and more boosters will become the norm.
Even with all the health misery, life disruption and economic devastation this pandemic is causing, there is absolutely no meaningful conversation about the common causes and origins of such disease, SARS, MERS, MERSA, etc…..factory farming and treatment of animals.
Yet public education and support for emphasizing fresh foods diet, de-emphasizing meat and prohibiting pandemic producing intensive industrial animal raising practices is not part of the discussion.
It’s well documented and basic knowledge that factory farming, intensive crowding is a peitrie dish for biological weapons and our level of consumption is unnecessary and unhealthy. In general, the public is trained (present NC company excluded) to focus symptoms rather than causes on many issues and I wonder if the human species is very inferior as most life forms quickly identify threats to existence if they are to survive.
yes, I’ve been half-expecting some new swine bug to hit during this. “how could we have known?”
Ping, thanks for good comment.
I have just bought and am reading “Dead Epidemiologists” by Rob Wallace, who goes into great detail about this very point, and the link between human communities and more “unspoilt, natural ” areas where movement of pathogens is generally able to be controlled by natural factors, but as we extend humanity and intensive agriculture into every nook and cranny, there are many ways for uncontrolled spread of pathogens.
I believe there is a mistake here:
I suppose it depends on your definition of ‘ultracold’ but
I spoke to a Doctor with a background in infectious disease and he is more confident in the mRNA candidates than the Astra Zeneca one: He said that mRNA research in vaccine development has been going on for a long time prior to the new vaccines and he believes based on what he knows that they appear to be effective and safe candidates for use. He was also concerned about the 62% effectiveness for the Astra Zeneca Vaccine with the larger dosage vs. the higher percentage with the smaller dosage.
Wired raises questions about the AZ vaccine. It looks like they had to move the goalposts to get the reported results…
” Instead, Oxford-AstraZeneca’s data came out of two separate studies: one in the UK that began in May, and another in Brazil, which got started at the end of June. These two studies were substantially different from one another: They didn’t have standardized dosing schemes across the trials, for one thing, nor did they provide the same “control” injections to volunteers who were not getting the experimental Covid vaccine. The fact that they may have had to combine data from two trials in order to get a strong enough result raises the first red flag.”
And some of the trials did not include anyone over 55. The article raises questions that seem well founded to me as a not-medical person.
https://www.wired.com/story/the-astrazeneca-covid-vaccine-data-isnt-up-to-snuff/
Both Time and Forbes magazine have articles detailing what appears to be a fundamental misunderstanding about what the mRNA clinical trials are actually measuring, vaccines not as a prophylactic against infection but used to alleviate symptoms in the already infected. From Forbes:
“One of the more immediate questions a trial needs to answer is whether a vaccine prevents infection. If someone takes this vaccine, are they far less likely to become infected with the virus? These trials all clearly focus on eliminating symptoms of Covid-19, and not infections themselves. Asymptomatic infection is listed as a secondary objective in these trials when they should be of critical importance.
It appears that all the pharmaceutical companies assume that the vaccine will never prevent infection. Their criteria for approval is the difference in symptoms between an infected control group and an infected vaccine group. They do not measure the difference between infection and noninfection as a primary motivation.”
From Time:
“It’s important to note what that effectiveness means. The results do not reflect complete or so-called sterilizing immunity against infection, but rather protection against COVID-19 illness and its serious consequences once someone has been infected. In fact, the study was not set up to test people regularly and compare those who were negative versus those who were positive in each of the vaccine and placebo groups. Instead, all of the participants were asked to report any symptoms of COVID-19 they experienced, including fever, shortness of breath, sore throats and intestinal problems. Once they reported symptoms, they were tested for SARS-CoV-2, either by requesting a swab to take a sample themselves, or by reporting to their trial site for a test. Researchers then looked at those who were confirmed to be infected, and compared disease outcomes among those getting the vaccine and those getting a placebo in this group.“
I hope a lot of people read your comment. it is critical to understand this.
but Time is wrong. they are not set up to measure effectiveness in terms of mortality
>>> However, an error in the early stages of the trial meant that some participants received only a half-dose in the first round. In the group of 2,741 volunteers who received a lower dose of the vaccine candidate followed a month later by a full booster dose, the efficacy was 90%, according to AstraZeneca. The efficacy was only 62% among the 8,895 volunteers who received both full doses.
To compute “efficacy”, one needs to know how many people received the vaccine, how many received placebo instead, and how many from each group actually got sick. For this calculation it is *critical* that these two sets of people are chosen at random *from the same population*. Same population means same distribution over time and same distribution over administration locations, among other things, because the virus prevalence ebbs and flows over time at different places.
So if the half-doses were administered during a different time extant and/or at different location(s) than the full-doses were, then each set of participants is part of a *different population*, and these should be treated as separate trials. So then I wonder if, when the researchers analyzed the data, did they lump all the placebo recipients together or did they (properly) divide the placebo members between these population two groups? Depending on the nature of the “error” that led to some vaccine recipients getting half-doses, it’s not clear the researchers would have enough information to do this division properly.
If their “efficacy” calculations for each are based on a lumped-together placebo group, then both reported results will have significant unknown errors. Now, I’d like to believe that the researchers would not make this mistake if they were able to avoid doing so and would say something if they couldn’t. However, I’m paranoid because even trained scientists are capable of making stupid errors like this unfortunately. Then there’s the enormous financial and political pressure to report positive results that might have motivated rushed reporting of that “90%” number even if someone there knew better.
Mind you, these reported efficacy numbers are nowhere near sufficient to judge the virtue of these vaccines against one another. It’s not just about differences in cost, storage requirements, side-effects, etc. It’s also about whether they convey actual immunity to *contagiousness* which may be very different from immunity to *COVID-19 positive status*. As I understand it, COVID-19 positive status requires both a positive test and symptoms from a rather short list. (I don’t even know if different researchers use the same symptom list.) So “COVID-19 positive status” likely only includes people with the full-blown disease, even though many people with “milder” symptoms or none at all might still be contagious. Some “negative” patients may also still develop long-COVID or undetected symptoms like heart damage.
To their credit, Moderna (but notably not Pfizer/BioNTech) have clarified that their data does not prove that their vaccine will stop spread, only that it protects the recipient from developing “significant” COVID-19 symptoms. Of course we’re still learning a lot about long-COVID including about symptoms that may not be obvious (and therefore “significant”) but may be no less serious in the long-run. Indeed, if long-COVID is more prevalent in the population than “significant” acute symptoms, it could be worse for society on-net. And to be extra paranoid, what if some of these vaccines *cause* long-COVID?
One last note about these announcements: I’m thinking that the hyping of these coming vaccines may be doing enormous short-term harm by causing people to behave less responsibly. The announcements may also cause politcos to feel less urgency about passing stimulus. People who read NC know this is completely illogical and insane, but we are a small minority. It really would help if more scientists and “scientific” authorities like Fauci, rather than rushing to declare “Mission Accomplished” would instead tamp down this enthusiasm and remind people that we still have a long hard struggle to go through, but unfortunately their public pronouncements seem to be motivated more by class-narcissism (and maybe literal profits in some instances like Fauci) than actual sound science. This is a failure of basic competence.
With regard to the first three paragraphs of my comment above: I see from Astrazeneca’s press release that they did divide the placebo group between the two populations. Hopefully they did this division correctly, so I apologize for jumping to conclusions on this.
With that said, the non-intuitive dose response data are very interesting, and I’d really like to know what kind of “serendipity” led to the half-dose error and what that might imply about the rest of the research process. Hopefully all is well, and they can later reproduce or improve on that 90% efficacy because this vaccine does look a lot better in most regards than the Pfizer and Moderna vaccines.
And now I see the comment above by marku52 and the link he posted from Wired (https://www.wired.com/story/the-astrazeneca-covid-vaccine-data-isnt-up-to-snuff/) which casts a lot of shade on this AZ vaccine and suggests that their whole “trial” was a whole hodgepodge of trials carried out with poor consistency between them. Of course the same article depicts the mRNA vaccines as untainted, despite the fact that we still only have press releases to judge them by as well.
What a righteous mess this is!
With all these trials, due to the race to beat the clock, it would seem to me there must be uncertainty about which of the different short-term vs long-term immunity mechanisms are acting to give the protection. But that’s an unavoidable limitation.
PS- the refrigeration isn’t the end of the world, you can ship in a double layers of dry ice and thick styrofoam insulation. Just costs a little more.
The Russian vaccine (Sputnik V) was developed at the Gamaleya Center, which is a world-class research institute. Both the Russian vaccine and also the Chinese vaccine, developed by Cansino, use an attenuated adenovirus vector, which is unable to replicate itself in the recipient. I feel safer with that method of inducing an immune response to the Corona virus spike protein than with the vector chosen by Oxford-Astrazeneca or either of the RNA vaccines. Next time I’m abroad, I’ll try to get vaccinated safely and effectively.
It’s pretty much policy in the west to ignore, downplay or demonize all things Russian, Iranian, Chinese etc. If it didn’t happen in, or it’s not developed by, a western country it will never get fair coverage in the media or be taken seriously.
The leaders of the decaying western empire have decided that a zero sum civilizational ‘great game’ between the west and the east is preferable to cooperation and a multipolar world. It’s hubristic insanity on a grand scale but supremacist ideologies don’t die easily.
I should note most of these vaccines will cross-react with the standard Abbot Laboratories HIV-ELISA, meaning anybody who gets them will get a false-positive HIV test for the rest of their lives. This was pointed out by Susan Buchbinder in a letter to the Lancet